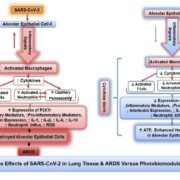
Introduction: The COVID-19 pandemic spread rapidly throughout the world, causing millions of infections, hundreds of thousands of deaths, and overloaded hospitals and intensive care units, with patients in need of critical care management. The initial hallmarks of COVID-19 were cytokine storm and acute respiratory distress syndrome (ARDS). Some patients are asymptomatic and recover spontaneously while others experience progressive symptoms leading to mild, moderate, or serious cases. Many serious cases require admission to intensive care units (ICUs) and ventilation support. The exceptional number of patients who died while receiving optimal medical care and ventilator support remains an enigma and successful treatment strategies remain to be found.
Case and outcomes: During the period from March 2020 to May 2020, we performed a preliminary clinical trial with a parallel design for the evaluation of PBMT on COVID-19 pneumonia (ClinicalTrials.gov identifier: NCT04391712). Before obtaining Institutional Review Board (IRB) approval, the US Food and Drug Administration assessed the MLS scanner-equipped laser and deemed it was a nonsignificant risk device. Subsequently, the IRB, and the Clinical Research Review Committee of the Lowell General Hospital (Massachusetts, USA) approved the clinical protocol for PBMT treatment of COVID-19 pneumonia. All patients provided written informed consent for participation in this trial. A preliminary 10 patient study was approved by the hospital. Patients were assigned to the PBMT group (standard medical care plus adjunctive PBMT) or control group (standard medical care) using the Sealed Envelope computer application (Table 1). There was no masking of the treatment group, and the study was performed in an open-label fashion. Inclusion criteria were: SARS-CoV-2 infection confirmed by nasopharyngeal swab and RT-PCR on an Abbott ID system upon hospitalization, age 18–90 years, and pulmonary compromise requiring oxygen support. Patients had to be able to self-prone or support themselves in a self-sitting position to facilitate the administration of PBMT. Exclusion criteria included patients who required ventilator management, those with autoimmune disorders or inflammatory conditions not related to COVID-19, and pregnancy. The trial was conducted in accordance with the Declaration of Helsinki.
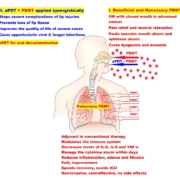
Conclusion: This study demonstrates the potential benefits of adjunctive PBMT in COVID-19 pneumonia. The use of PBMT in the early stages of severe ARDS in COVID-19 patients may improve pulmonary and clinical status and reduce the need for ventilator support and ICU stay. Adjunctive PBMT may decrease hospital stay and enhance the recovery process. Clinical status improvement was supported by an increase in SpO2 during the treatment sessions, the rapid relief of respiratory symptoms, and improved CXR findings. There was no incidence of mortality or major reported side effects in the PBMT group. The mortality rate was 40% in the control group and 40% of patients continue to experience pulmonary sequelae. The patients in the PBMT group recovered without needing ICU admission or mechanical ventilation. Conversely, 60% of patients in the control group required ICU admission and ventilation. Clinical trials with larger group sizes are necessary to confirm the effects of PBMT on COVID-19 pneumonia
READ MORE